Photo Dynamic Compounds
The future of anti-cancer therapy
What is Theralase® Anti-Cancer Therapy?
Anti-Cancer Therapy ("ACT") uses Photo Dynamic Compounds ("PDCs") (light-sensitive compounds) that when “activated” by laser light are able to produce a violent form of oxygen known as singlet oxygen or Reactive Oxygen Species (“ROS”), which causes oxidative stress to cancer cells and forces the cancer cell through Immunogenic Cell Death ("ICD").
Theralase® has optimized and modernized traditional PDT, strengthening the potential to treat a broad range of diseases and overcome the limitations of current cancer treatment methodologies.
Theralase® is currently evaluating ACT for Non-Muscle Invasive Bladder Cancer (“NMIBC”). The Company is also conducting scientific and preclinical research in Glio Blastoma Multiforme (“GBM”) (a deadly form of brain cancer) and Non-Small Cell Lung Cancer (“NSCLC”).
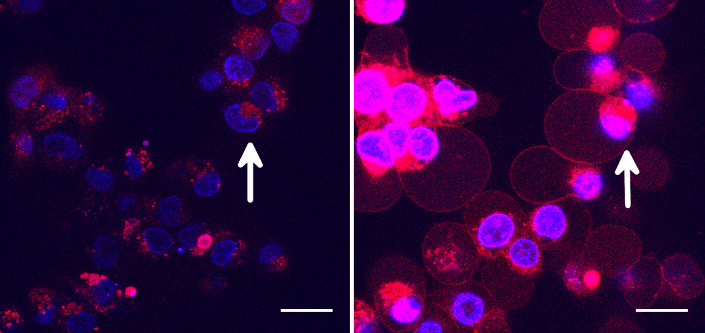

NMIBC Study Drug TLD-1433
What are Photo Dynamic Compounds?
PDCs are light-sensitive drugs that are cytotoxic (harmful to cells) when exposed to light at specific wavelengths and power levels.
Theralase®’s PDCs are unique; in that they can be activated by a wide range of laser wavelengths and powers, regardless of the oxygenation level present in the tissue under treatment; a unique and advantageous feature when dealing with cancer cells that thrive in low oxygenated tissues, such as solid core tumours (i.e.: brain, breast, lung, bladder cancers).
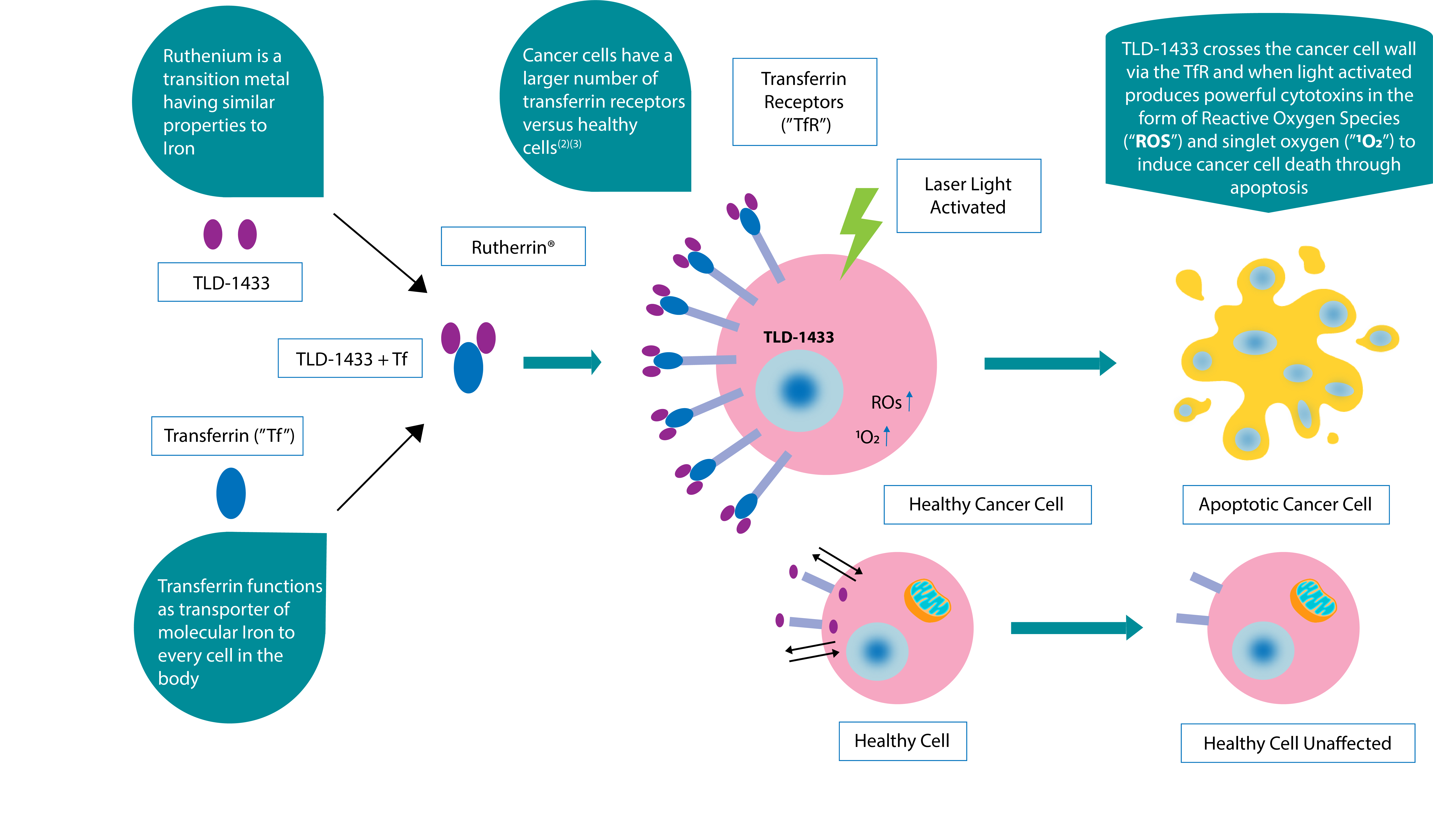
Theralase®’s lead compound is a ruthenium based molecule, known as TLD-1433, which when combined with a endogenous human glycoprotein, known as transferrin, becomes the patented compound Rutherrin®.
TLD-1433 has been demonstrated preclinically and clinically to be preferentially absorbed into bladder cancer cells, leaving healthy cells virtually unscathed. After entering a cancer cell and becoming light activated, TLD-1433 has been demonstrated to be highly toxic to cancer cells, producing > 99.9% kill rate at nano molar concentrations.
NMIBC Study Drug TLD-1433
TLD-1433 Mechanism of Action(1)

1 Kaspler P, Lazic S, Forward S, Arenas Y, Mandel A, Lilge L. A ruthenium(ii)based photosensitizer and transferrin complexes enhance photo-physical properties, cell uptake, and photodynamic therapy safety and efficacy. Photochem Photobiol Sci. 2016 Apr;15(4):481-95. doi: 10.1039/c5pp00450k. Epub 2016 Mar 7. PubMed PMID: 26947517.
2 Transferrin receptor regulates pancreatic cancer growth by modulating mitochondrial respiration and ROS generation. Jeong SM, Hwang S, Seong RH
3 A novel transferrin receptor-targeted hybrid peptide disintegrates cancer cell membrane to induce rapid killing of cancer cells. Megumi Kawamoto, Tomohisa Horibe, Masayuki Kohno,Koji Kawakami
Theralase® has optimized and modernized traditional PDCs, strengthening the potential to treat a broad range of diseases and overcome the limitations of current cancer treatment methodologies
ACT is a promising strategy for the treatment of cancer; whereby, light is used to activate an otherwise nontoxic Photo Dynamic Compound (“PDC”) in order to primarily destroy the cancer cells and secondarily to induce an immune response. Following photoactivation, the PDC delivers a toxic burst of cytotoxic singlet oxygen and other Reactive Oxygen Species ("ROS") that are confined spatially and temporally to the irradiated region, thus targeting malignant tissue while sparing healthy tissue.
An ideal PS should:
- Exhibit PDT efficacy at low concentrations (high efficacy, low toxicity)
- Have high PDT efficacy in the presence of light (light toxicity) and have no efficacy without the presence of light (dark toxicity) (high light toxicity, low dark toxicity)
- Be soluble in aqueous solvents (water soluble)
- Exhibit an oxygen-independent Mechanism Of Action (“MOA”) for treating hypoxic tissue, such as solid core tumours (i.e.: brain, breast, lung, bladder cancers) (no need for molecular oxygen to work)
- Be effective in a wide range of wavelengths (red light, as well as Near Infra Red (“NIR”) light) (allows activation at various tissue depths)
The Theralase PDC, TLD-1433, is a metal-based compound derived from organic ligands chelated to the metal atom ruthenium (“Ru”).
The resulting molecular structure allows for:
- Absorption of light in a wide wavelength range in a biological context, from ultraviolet (“UV”) through the visible spectrum to NIR light
- Solubility and stability in water
- Resistance to photobleaching (retaining its PDC properties over extended light exposure times, allowing for longer treatments without the need for reapplication)
- Singlet oxygen quantum yield near unity (resulting in efficient conversion of photons to cytotoxic ROS)
- Activation via both Type I (oxygen independent) and Type II (oxygen dependent) processes (allowing it to be a versatile PS in oxygen rich and/or poor environments)
As TLD-1433 absorbs in green, red, and NIR light in a biological context, it is a versatile PDC, with the ability to adjust the tissue treatment depth by varying the treatment wavelength(s). TLD-1433 mediated PDT treatment of bladder carcinoma is performed with green light irradiation, ensuring only the superficial bladder layers are treated, thus preventing deep tissue or musculature damage.
The study device (TLC-3200) utilizes a laser emitter and detector in combination with the study drug, TLD-1433, during the study treatment to destroy bladder cancer following Trans-Urethral Resection of the Bladder Tumour (“TURBT”).
The study device uses real tissue feedback dosimetry to monitor for safety and assist the Principal Investigator (“PI”) in the delivery of laser light to the inner surface bladder wall; therefore, the PI is alerted to warnings / errors during the administration of the study treatment, allowing the PI to adjust the physical location of the emitter and / or detector or to replace it altogether, depending on the warning / error message, increasing the patient safety profile.






The Advantage and Benefits of Photo Dynamic Compounds
No long-term Adverse Events (“AEs”), compared to surgery, radiation and/or chemotherapy
Less invasive than surgery
Study Treatment is a “Two and Done”, Study Treatment (2 Study Treatments performed approximately 6 months apart)
Precise targeting of cancerous cells, leaving healthy cells intact
Unlike radiation, PDT can be repeated many times to the same location, if required
Little to no scarring
Proven safe and effective, in a Phase Ib clinical study
IMPORTANT INFORMATION FOR PATIENTS
If you are a patient interested in participating in the Phase II NMIBC clinical study (“Study II”), please feel free to contact Theralase® or the nearest participating clinical study site for more information.
If enrolled in Study II, there is a no charge for participation in Study II, however, your urologist or family physician will need to contact the principal investigator to provide confidential information about your NMIBC to ensure you meet the clinical study guidelines.
For patients or healthcare practitioners wishing to learn more about Study II, please visit ClinicalTrials.gov and search for "Theralase" or by the National Clinical Trial Number: NCT03945162.