TORONTO, ON / ACCESSWIRE / September 4, 2019 / Theralase® Technologies Inc. (“Theralase” or the “Company”) (TSXV:TLT) (OTCQB:TLTFF), a clinical stage biopharmaceutical company developing Anti-Cancer treatments with their light activated Photo Dynamic Compounds (“PDC”) and associated drug formulations, announced today that, the first patient has been treated in its Phase II clinical study titled “A Phase II Clinical Study of Intravesical Photodynamic Therapy (“PDT”) in Patients with Bacillus Calmette Guérin (“BCG”) Unresponsive Non-Muscle Invasive Bladder Cancer (“NMIBC”) or Patients who are Intolerant to BCG Therapy (“Study II”)” at University Health Network (“UHN”) in Toronto.
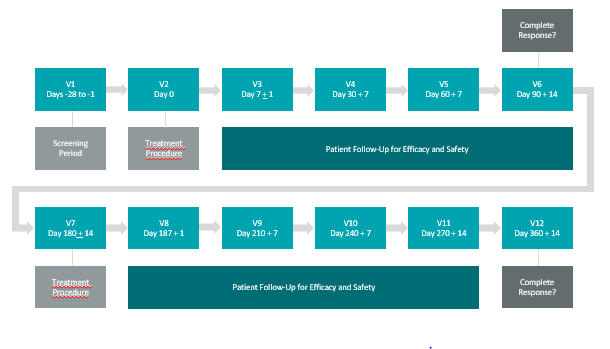
This marks the official launch of Study II which focuses on the treatment of approximately 100 BCG-Unresponsive NMIBC patients presenting with Carcinoma In-Situ (“CIS”)in approximately 20 clinical study sites located in Canada and the US, with a primary endpoint of efficacy at any point in time, measured by Complete Response (“CR”), a secondary endpoint of duration of CR and a tertiary endpoint of safety.
Arkady Mandel, M.D., Ph.D., D.Sc., Chief Scientific Officer, Theralase stated that, “Initiation of patient treatment in our Phase II study is a significant milestone for our Photo Dynamic Therapy (“PDT”) research and development programs. NMIBC is a recurrent and progressive cancer, which Theralase believes it’s TLD-1433 therapy can make a significant difference in. Current standards of care for NMIBC including BCG, chemotherapy and immunotherapy, are not curative in nature and unfortunately are associated with considerable morbidity. This underscores the urgent need for new treatment options like Theralase’s TLD-1433 PDT for patients contending with this debilitating and fatal disease.”
Shawn Shirazi, Ph.D., CEO – Drug Division, Theralase stated that, “Treating patient one in a pivotal Phase II clinical study commences an exciting journey for the Company aimed to demonstrate to both the public and the scientific community what Theralase is capable of. Our clinical research team is working diligently on patient enrollment and on-boarding of additional Canadian and US study sites, subject to regulatory approval. If Study II can duplicate the efficacy results demonstrated in the Phase 1b NMIBC Clinical Study, Theralase will have an opportunity to be the next gold standard for patients with NMIBC, subject to regulatory approval.”
About Study II
The primary and secondary endpoint will be evaluated by:
CR in patients with CIS with resected papillary disease at any time point post-treatment with a duration of CR evaluated at approximately 360 days post-treatment.
Patient CR is defined as one of the following (no cancer detected in bladder):
- Negative cystoscopy and negative (including atypical) urine cytology (no cancer detected in urine)
- Positive cystoscopy (cancer detected in bladder) with biopsy-proven benign or low-grade NMIBC
- Negative cystoscopy with malignant urine cytology (no cancer detected in urine), if cancer is found in the upper tract or prostatic urethra and random bladder biopsies are negative
The tertiary endpoint will be evaluated by:
Incidence and severity of Adverse Events (“AEs”) Grade 4 or higher that do not resolve within 360 days post-treatment; whereby: Grade 1 = Mild, Grade 2 = Moderate, Grade 3 = Severe, Grade 4 = Life-threatening or disabling, Grade 5 = Death
About NMIBC:
In 2019, an estimated 80,470 adults (61,700 men and 18,770 women) were diagnosed with bladder cancer in the United States. Among men, bladder cancer is the fourth most common cancer. It is estimated that 17,670 deaths (12,870 men and 4,800 women) from this disease will occur in 2019. Among men, bladder cancer is the eighth most common cause of cancer death.1 The bladder cancer market is expected to triple in size to around $1.1 billion in 2025.2
About Theralase® Technologies Inc.
Theralase® is a clinical stage pharmaceutical company dedicated to the research and development of light activated Photo Dynamic Compounds and their associated drug formulations intended to safely and effectively destroy various cancers.
Additional information is available at www.theralase.com and www.sedar.com
1 Cancer Facts and Figures 2019. American Cancer Society. https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2019.html. Accessed January 14, 2019.
2 Bladder cancer market size to more than triple to over $1.1 billion by 2025. (2017). Retrieved 14 August 2019, from https://www.globaldata.com/bladder-cancer-market-size-triple-1-1-billion-2025/
Forward Looking Statement:
This news release contains “forward-looking statements” which reflect the current expectations of management of the Company’s future growth, results of operations, performance and business prospects and opportunities. Such statements include, but are not limited to, statements regarding the Company’s proposed development plans with respect to Photo Dynamic Compounds and their drug formulations. Wherever possible, words such as “may“, “would“, “could“, “should”, “will“, “anticipate“, “believe“, “plan“, “expect“, “intend“, “estimate“, “potential for” and similar expressions have been used to identify these forward-looking statements. These statements reflect management’s current beliefs with respect to future events and are based on information currently available to management. Forward-looking statements involve significant risks, uncertainties and assumptions including with respect to the ability of the Company to: adequately fund, secure the requisite regulatory approvals to commence and successfully complete a Phase II NMIBC clinical study in a timely fashion and implement its development plans. Many factors could cause the Company’s actual results, performance or achievements to be materially different from any future results, performance or achievements that may be expressed or implied by such forward-looking statements; including, without limitation, those listed in the filings made by the Company with the Canadian securities regulatory authorities (which may be viewed at www.sedar.com). Should one or more of these risks or uncertainties materialize or should assumptions underlying the forward looking statements prove incorrect, actual results, performance or achievements may vary materially from those expressed or implied by the forward-looking statements contained in this news release. These factors should be considered carefully and prospective investors should not place undue reliance on the forward-looking statements. Although the forward-looking statements contained in the press release are based upon what management currently believes to be reasonable assumptions, the Company cannot assure prospective investors that actual results, performance or achievements will be consistent with these forward-looking statements. The Company disclaims any intention or obligation to revise forward-looking statements whether as a result of new information, future developments or otherwise except as required by law. All forward-looking statements are expressly qualified in their entirety by this cautionary statement.
Neither TSX Venture Exchange nor its Regulation Services Provider (as that term is defined in the policies of the TSX Venture Exchanges) accepts responsibility for the adequacy or accuracy of this release.
For More Information:
1.866.THE.LASE (843-5273)
416.699.LASE (5273)
Shushu Feng, Investor Relations & Public Relations Coordinator
sfeng@theralase.com
Amelia Tudo, Investor Relations & Public Relations Coordinator
atudo@theralase.com
www.theralase.com