News and Press Releases
Press releases
Theralase® Extends Warrants
Press ReleaseAug 29, 2025 4:30 PM EDT Toronto, Ontario–(Newsfile Corp. – August 29, 2025) – Theralase® Technologies Inc. (TSXV: TLT) (OTCQB: TLTFF) (“Theralase®” or the “Company“), a clinical stage pharmaceutical company pioneering light, radiation...
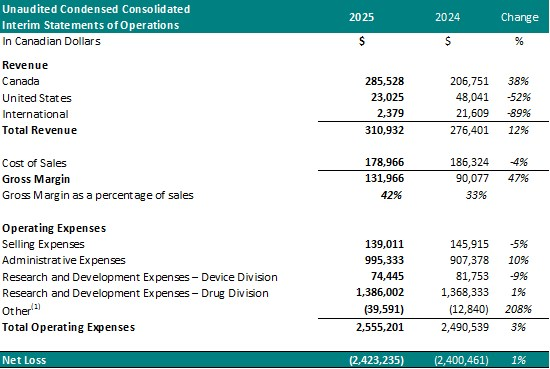
Theralase® Releases 2Q2025 Financial Statements
Press ReleaseToronto, Ontario–(Newsfile Corp. – August 26, 2025) – Theralase® Technologies Inc. (TSXV: TLT) (OTCQB: TLTFF) (“Theralase®” or the “Company“), a clinical stage pharmaceutical pioneering light, radiation, sound and drug-activated therap...
Theralase® Extends Warrants
Press ReleaseTheralase® Technologies Inc. plans to extend the expiry date of 4,800,000 share purchase warrants from June 30, 2025, to June 30, 2028. The warrants, issued in 2023, remain exercisable at $0.35 per share under the same terms. The...
Theralase® Closes Non-Brokered Private Placement
Press ReleaseTheralase® Technologies Inc. closed a $571,000 private placement, issuing 2,855,000 units at $0.20 each. Funds will support its Phase II bladder cancer trial, Rutherrin® development, and corporate needs. Each unit includes a share and...
Theralase® Completes Annual General and Special Meeting
Press ReleaseTheralase® successfully completed its 2025 Annual General Meeting, sharing strategic plans for 2025–2026 focused on clinical advancements and commercialization. A recording of the presentation is available for shareholders....
Theralase® Annual General Meeting
Press ReleaseTheralase® Technologies Inc. reminds shareholders of its Annual General and Special Meeting (AGSM) on June 11, 2025, at 4:30 pm ET in Toronto. Following the meeting, a virtual corporate presentation and Q&A session will be held at 5:15...
Theralase® 1Q2025 Financial Statements
Press ReleaseTheralase® reported Q1 2025 results showing lower revenue and increased R&D expenses, ongoing Study II enrollment with promising bladder cancer interim data, continued HSV treatment development, and plans for Rutherrin® clinical trials...
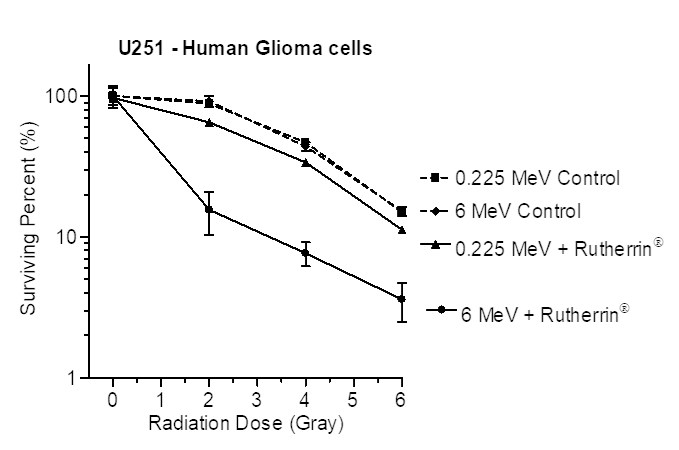
CORRECTION FROM SOURCE: Theralase® to Present Groundbreaking Research at ASTRO 2025
Press ReleaseTheralase® will present preclinical data comparing radiation-activated Rutherrin® versus radiation alone at ASTRO 2025, highlighting tumor targeting, immune activation, and survival benefits; clinical trials for various cancers begin...
Theralase® to Present Groundbreaking Research at ASTRO 2025
Press ReleaseTheralase®’s Rutherrin® is 100x more effective than radiation alone in preclinical cancer models, showing tumor targeting, immune activation, and improved survival; Phase I/II clinical trials start early 2026 targeting multiple cancers....
Theralase® Provides Corporate Update
Press ReleaseTheralase advances Ruvidar® trials for bladder and other cancers, targeting NDA submissions by 2026, launching multiple Phase I/II studies, seeking US listing, and pursuing international partnerships for commercialization and research...
in the news
July 6, 2023
Vrian Crombie Radio Interview with Roger DuMoulin-White - President and Chief Executive Officer
may 18, 2023
Promising Developments in the Theralase® Project of Intravesical Photodynamic Therapy for BCG Unresponsive Bladder Cancer - Girish Kulkarni