News and Press Releases
Press releases
London Health Sciences Centre Launches as Newest Clinical Study Site for Phase II Non-Muscle Invasive Bladder Cancer Clinical Study
Press ReleaseToronto, Ontario – October 07, 2019, Theralase® Technologies Inc. (“Theralase” or the “Company”) (TSXV: TLT) (OTCQB: TLTFF), ), a clinical stage pharmaceutical company dedicated to the research and development of light activat...
Theralase Files Investigational New Drug Application with the FDA
Press ReleaseToronto, Ontario – October 01, 2019, Theralase® Technologies Inc. (“Theralase” or the “Company”) (TSXV: TLT) (OTCQB: TLTFF), a clinical stage pharmaceutical company focused on the research and development of light activated Ph...
Patient Six Cancer-Free Eighteen Months After Single Anti-Cancer Treatment
Press ReleaseToronto, Ontario – September 30, 2019, Theralase® Technologies Inc. (“Theralase” or the “Company”) (TSXV: TLT) (OTCQB: TLTFF), a clinical stage pharmaceutical company dedicated to the research and development of light activat...
Theralase Grants Stock Options
Press ReleaseTORONTO, ON / September 13, 2019 / Theralase® Technologies Inc. (“Theralase” or the “Company”) (TSXV:TLT) (OTCQB:TLTFF), a clinical stage biopharmaceutical company developing Anti-Cancer treatments with their light activated Pho...
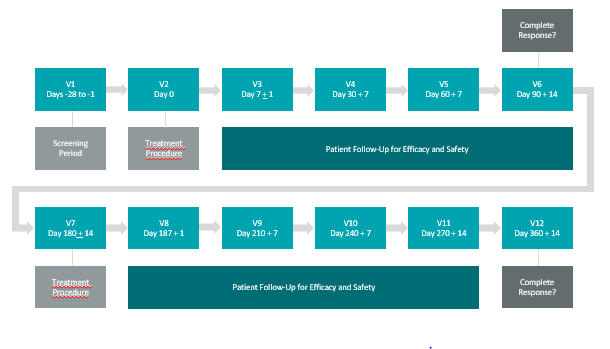
Theralase Annouces First Patient Treated in Phase II Non-Muscle Invasive Bladder Cancer Clinical Study
Press ReleaseTORONTO, ON / ACCESSWIRE / September 4, 2019 / Theralase® Technologies Inc. (“Theralase” or the “Company”) (TSXV:TLT) (OTCQB:TLTFF), a clinical stage biopharmaceutical company developing Anti-Cancer treatments with their light a...
Theralase Releases Second Quarter 2019 Financial Results and Company Update
Press ReleaseTORONTO, ON/ August 29, 2019/ Theralase® Technologies Inc. (“Theralase” or “Company“) (TSXV: TLT) (OTCQB: TLTFF), a clinical stage pharmaceutical company dedicated to the research and development of light activated Photo Dynami...
THERALASE ANNOUNCES CLOSING OF PROSPECTUS OFFERING FOR GROSS PROCEEDS OF $17,250,000
Press ReleaseNOT FOR DISTRIBUTION TO UNITED STATES NEWSWIRE SERVICES OR FOR DISSEMINATION IN THE UNITED STATES Toronto, Ontario – August 22, 2019, Theralase® Technologies Inc. (“Theralase” or “Company”) (TSXV: TLT) (OTCQB: TLTFF), is pleas...
Patient Five Cancer-Free Eighteen Months After Single Anti-Cancer Treatment
Press ReleaseToronto, Ontario – July 31, 2019, Theralase Technologies Inc. (“Theralase®” or the “Company”) (TSXV: TLT) (OTCQB: TLTFF), a clinical stage pharmaceutical company dedicated to the research and development of light activated Pho...
McGill University Health Centre Newest Clinical Study Site for Phase II Non-Muscle Invasive Bladder Cancer Clinical Study
Press ReleaseToronto, Ontario –July 30, 2019, Theralase® Technologies Inc. (“Theralase” or the “Company”) (TLT:TSXV) (TLTFF:OTCQB), a clinical stage pharmaceutical company dedicated to the research and development of light activated Photo ...
Theralase Signs Agreement with Urology Organization to Provide US Clinical Study Sites for its Phase II Non Muscle Invasive Bladder Cancer Clinical Study
Press ReleaseToronto, Ontario – July 24, 2019, Theralase® Technologies Inc. (“Theralase” or the “Company”) (TSXV: TLT) (OTCQB: TLTFF), a clinical stage pharmaceutical company dedicated to the research and development of light activated Pho...
in the news
July 6, 2023
Vrian Crombie Radio Interview with Roger DuMoulin-White - President and Chief Executive Officer
may 18, 2023
Promising Developments in the Theralase® Project of Intravesical Photodynamic Therapy for BCG Unresponsive Bladder Cancer - Girish Kulkarni