Smart Laser Technology
Therapeutic Laser Series TLC-2000
SMART LASER TECHNOLOGY
Setting New Standards for Therapeutic Laser Innovations
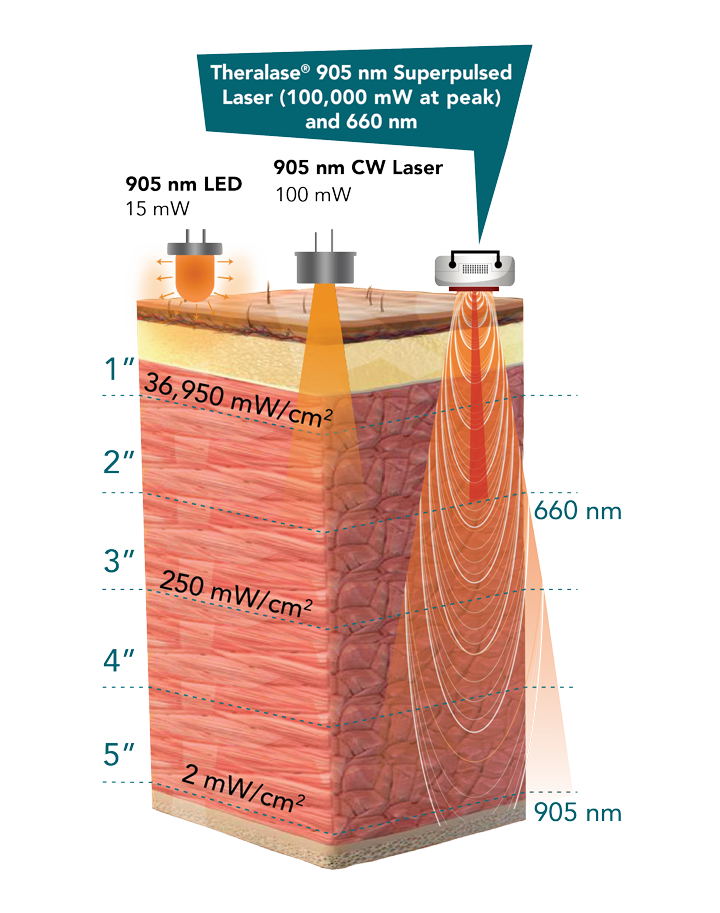
The Theralase® TLC-2000 CLT is a medical device consisting of a hand-held multiple laser probe (5 x 905 nanometer (“nm”) Near Infra Red (“NIR”) super-pulsed laser diodes, providing up to 200 milliWatts (“mW”) average power each, superpulsed at 100 Watts and 4 x 660 nm visible red laser diodes, providing up to 100 mW average power each, pulsed at 300 mW). The TLC-2000 CLT is used in direct contact with tissue to deliver laser light energy deep into tissue.

Super-pulsed, Multi-wavelength And Revenue-generating Technology ("SMART")
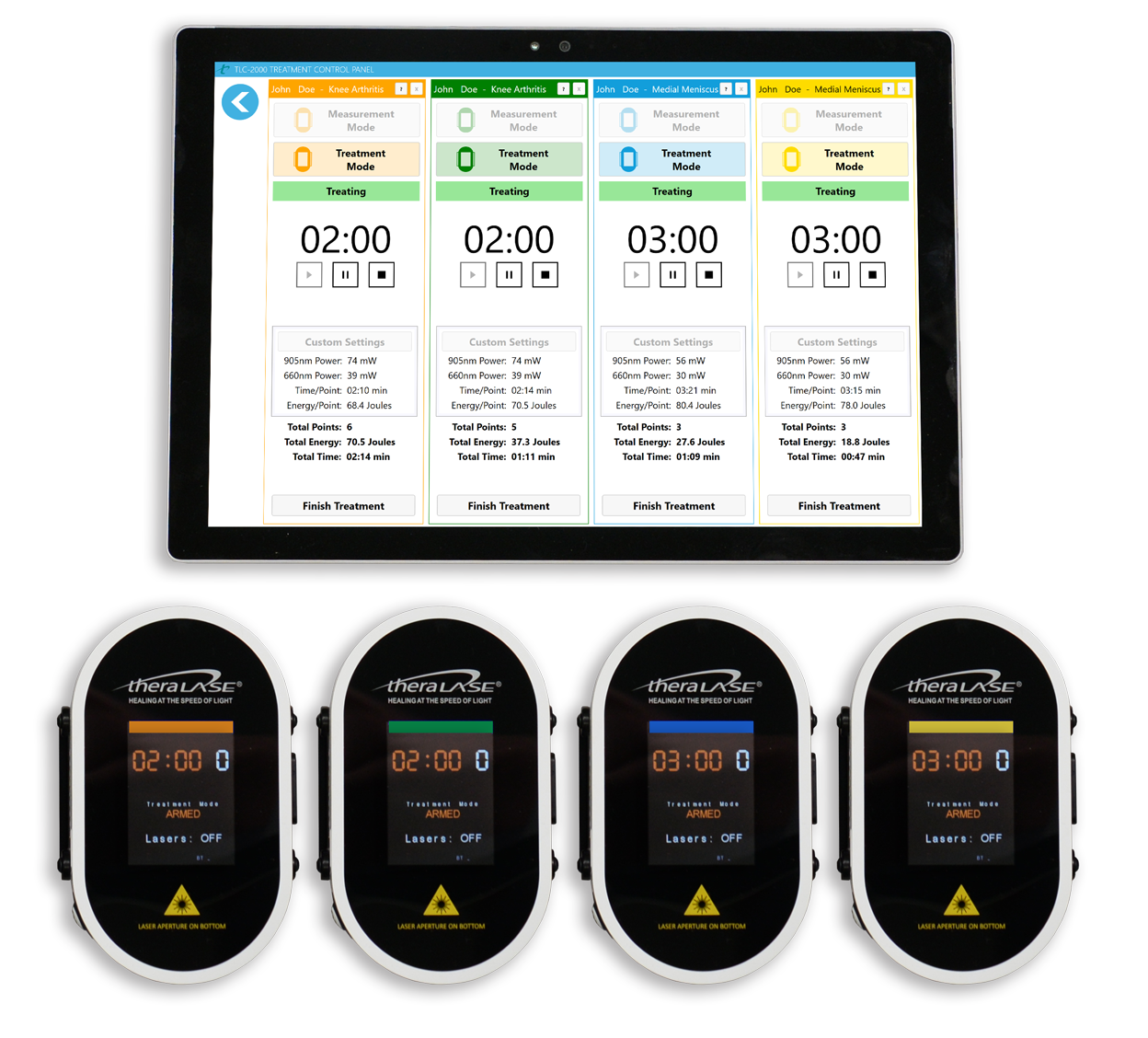
Theralase® SMART CLT is a first of its kind. An approach that features a patient-focused algorithm that, with the help of wireless BlueTooth® technology, can treat up to 4 patients or 4 different patient conditions, simultaneously, using only true laser diodes and a superpulsed delivery mode to accelerate healing, reduce inflammation and eliminate pain.
Features and Benefits



Customize Protocols - Get More Effective Results
Theralase® CLT emits laser light at specific visible and NIR wavelengths designed to stimulate biological reactions on the cellular level that are able to: eliminate pain, reduce inflammation and accelerate healing; however, the ability to deliver the optimal dose of laser light energy to the injured tissue location can be difficult, as patients have various physical characteristics (shapes, size and skin colouration), all of which affect the way laser light energy is absorbed and transmitted through the body.
Theralase®’s TLC-2000 CLT is able to adjust clinical protocol settings at the tissue surface to maintain and deliver the intended clinical energy density at tissue depth, independent of the patient’s physical characteristics, ensuring that patients receive the maximum therapeutic benefit from this innovative treatment.
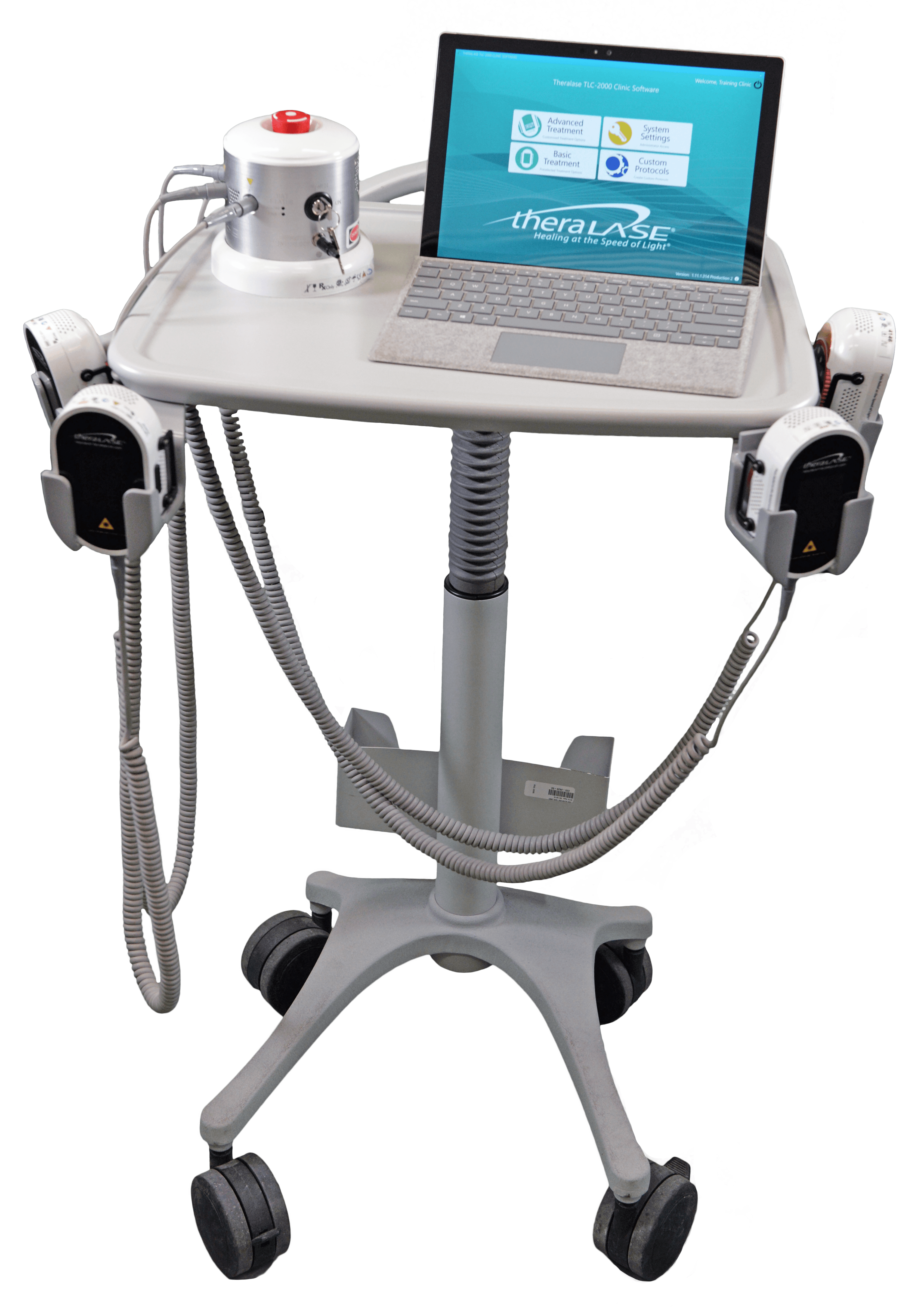
TLC-2000 Configurations
Theralase® superpulsed 905 nm NIR and 660 nm visible red laser technology accelerates healing by reducing pain and inflammation while staying below the Maximal Permissible Exposure (“MPE”) limit for tissue. Each treatment is able to be customized per patient to ensure that they are receiving the optimal dosage of laser energy. To achieve this, Theralase® CLT systems employ customizable pre-programmed treatment protocols that adjust using individual patients' physical characteristics and biometric information. Using superpulsed laser technology, Theralase® systems are able to penetrate up to 5 inches of tissue depth.
TLC-2100 system


TLC-2200 system


TLC-2300 system


TLC-2400 system






System Specifications

Additional Accessories


Additional LASER Probe
To optimize an existing TLC-2000 CLT system, an additional laser probe (up to 4) allows you to treat more than one condition or more than one patient simultaneously, significantlyincreasing revenue and patient satisfaction, while reducing healthcare practitioner time.


Additional computer
Adding an additional computer to an existing TLC-2000 CLT system allows you to streamline your treatments by having front-desk staff enter patient data prior to treatment, maximizing the productivity of your healthcare practice.


Power Pack
Adding a second power pack to your TLC-2000 CLT system allows you to expand your laser therapy practice and treat patients in multiple locations within your clinic.


Straps
Adding additional straps to your TLC-2000 CLT system allows hands-free applications when treating your patients.


Hard Carrying Case
This indestructible and waterproof medical laser case protects your Theralase® system from damage during transport.
Dimensions: 18.06” x 12.89” x 6.72” (45.9 cm x 32.7 cm x 17.1 cm).


Laser Safety Eyewear
Laser safety eyewear protects patients and practitioners from 660 nm and 905 nm laser light during treatment. procedures


Laser Safety Eye Cups
Laser safety eye cups are designed to cover the eyes to ensure zero light penetration, when treating around the facial region.


Hair Separator
A versatile accessory, the hair separator ensures that all energy being transmitted by your TLC-2000 CLT system reaches the surface of the skin, leading to improved outcomes for your patients.


Probe Holder *NEW*
Probe holders conveniently support your TLC-2000 CLT system laser probes and provide you with easy access to the tools you need to deliver CLT treatments to your patients.


Transport Stand
This transport stand is able to hold all of your TLC-2000 CLT accessories, and provides added convenience, organization, and flexibility to your treatment space.


Manuals
Theralase® TLC-2000 CLT protocol manuals provide quick reference guides and detailed treatment protocols for human, companion animal and equine patients.